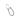Types of testing for the state registration of medical devices
Assessment of the device's compliance with the technical requirements declared by the manufacturer.
Technical testing
Assessment of the device's biological compatibility with human tissues and organs (skin, mucous membranes, internal organs).
Toxicological studies
Study of the safety and efficacy of the device when used on humans.
Clinical trials
Determination of the metrological characteristics of medical devices, including the accuracy of indicators in units approved for use in the Russian Federation.
Testing applies to medical devices classified as measuring instruments.
Testing applies to medical devices classified as measuring instruments.
Testing for type approval of measuring equipment
Regulatory and legislative documents for the procedure
The procedure for conducting technical testing, toxicological studies, and clinical trials for the state registration of medical devices is approved by Order No. 885n of the Ministry of Health of Russia dated August 30, 2021.
The procedure for conducting testing and the list of medical devices classified as measuring instruments are approved by Order No. 89n of the Ministry of Health of Russia dated August 15, 2012.
Procedure timelines
The timelines for conducting testing depend on the type of device, risk class, and complexity of the studies. During the testing process, the need for additional research or documentation adjustments may arise, which could extend the timelines. To expedite the registration process, some types of testing may be conducted in parallel.
RZN.Expert services for organizing and supporting medical device testing
We offer services for organizing and supporting medical device testing: from selecting a contractor to preparing the documents required for the registration dossier.
Determining the types of required testing. Assessing the readiness of the manufacturer (producer) to conduct testing.
Consultation
01
- Selection of an accredited organization for conducting testing.
- Preparation of the required documentation.
- Collection of samples and delivery to the laboratory (if required).
- Development of a clinical trial plan.
- Preparation of the required documentation.
- Collection of samples and delivery to the laboratory (if required).
- Development of a clinical trial plan.
Organization of tests: technical, clinical, toxicological or for the approval of types of means
02
- Organizing interaction among all process participants.
- Monitoring the testing process.
- In some cases, testing may be conducted by our company.
- Monitoring the testing process.
- In some cases, testing may be conducted by our company.
Conducting testing and monitoring
03
- Collection of research results, evaluation of the obtained data, including preparation and proofreading of final versions of the clinical trial protocol, and making necessary adjustments to the protocol.
- Preparation of documentation based on the testing results for the registration dossier, ensuring compliance with all requirements of Roszdravnadzor.
- Preparation of documentation based on the testing results for the registration dossier, ensuring compliance with all requirements of Roszdravnadzor.
Collection and preparation of reporting documentation
04
RZN.Expert offers its clients both one-time services for organizing specific types of medical device testing and comprehensive solutions that include the entire testing cycle and obtaining the necessary approvals.
Our company's experts successfully help clients minimize the risks of registration refusal and reduce the timelines for medical device registration.
Our company's experts successfully help clients minimize the risks of registration refusal and reduce the timelines for medical device registration.
05
Other services within the scope of medical device registration
RZN Expert provides services for QMS analysis and implementation in compliance with ISO 13485 requirements for medical devices. We conduct an in-depth assessment of your processes, identify vulnerabilities, and develop tailored solutions for improvement.
Our experts will help you not only obtain ISO 13485 certification but also implement safe and effective procedures that meet global standards. We ensure full compliance with all medical device quality management system requirements, opening new opportunities for international market entry and increasing trust in your products.
RZN Expert also offers a full range of services for medical device registration with Roszdravnadzor.
Our experts will help you not only obtain ISO 13485 certification but also implement safe and effective procedures that meet global standards. We ensure full compliance with all medical device quality management system requirements, opening new opportunities for international market entry and increasing trust in your products.
RZN Expert also offers a full range of services for medical device registration with Roszdravnadzor.
m. Kropotkinskaya
Moscow, Voekhonka Street, 15
Our Medtech Business information resource on telegram. Legislative changes, market overview and science in the field of medical devices.